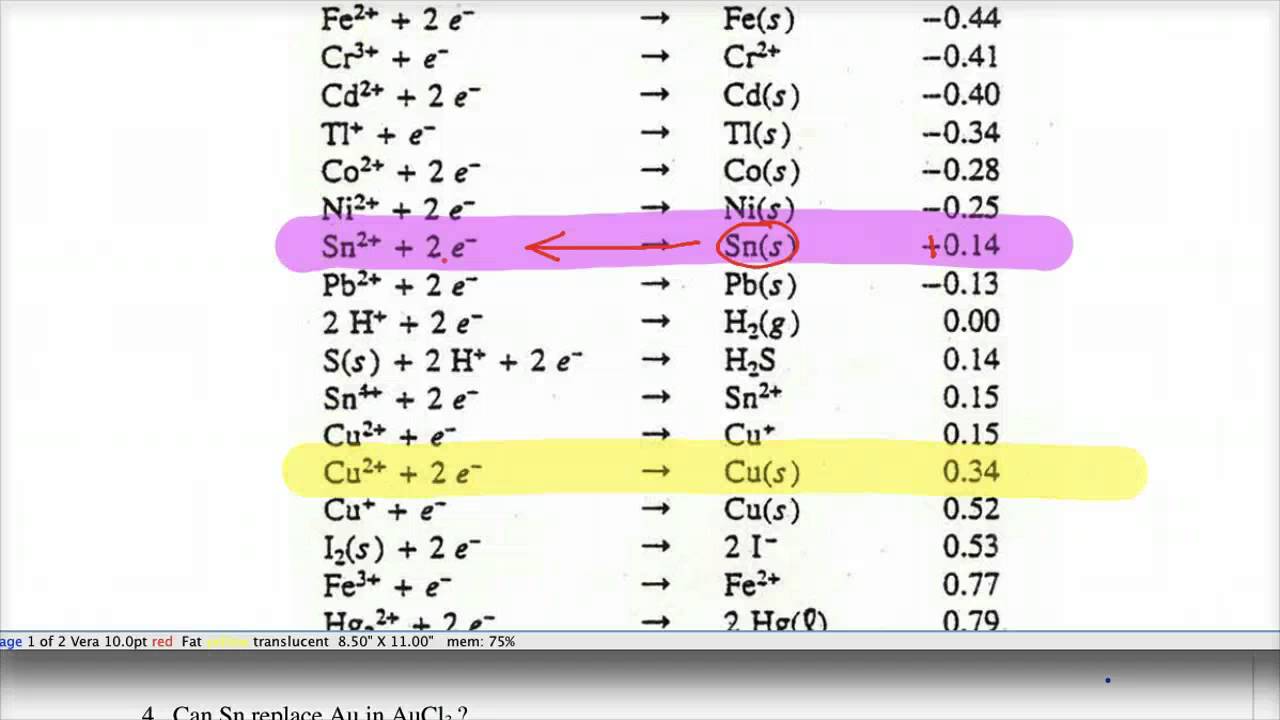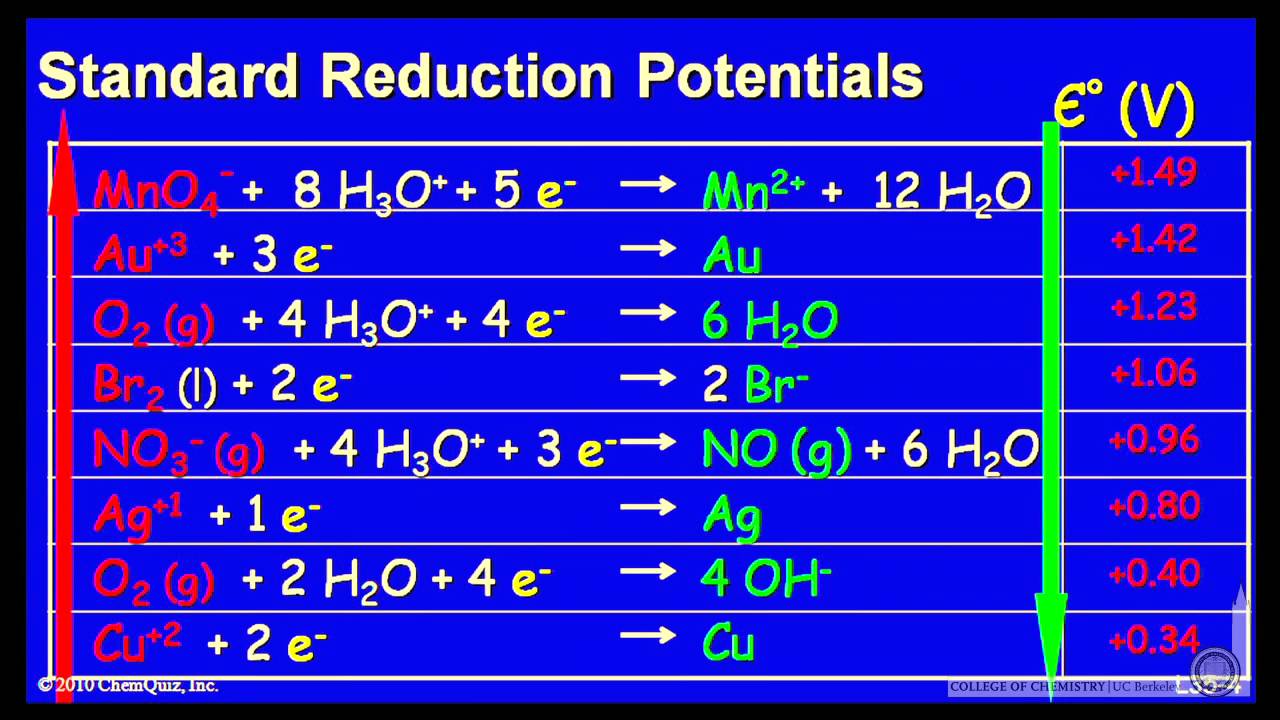
SOLVED: TABLE 18.1 Standard Reduction Potentials at 25 cC Reduction half-reaction Fi(g) (0q MnO (aq 8H+(aq) E'(V) 32F-(aq) 3Ce-(aq) 3Mn?-(aq) 4H,o(e) 2CI-(aq) 72H-O(Q) 2Br (aq) NO(g) ` 2H,O(Q) Ag(s) 3Fe-+(aq) 721-(aq) 3Culs)

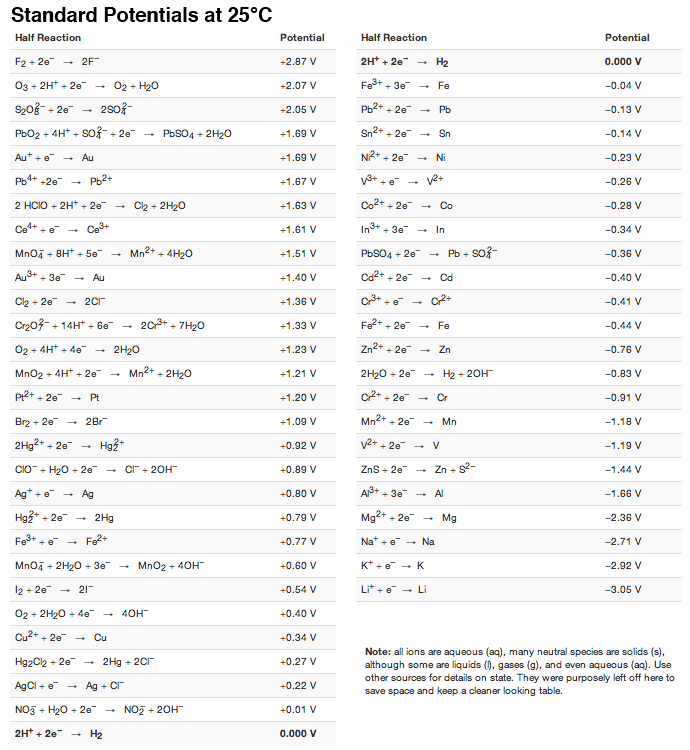
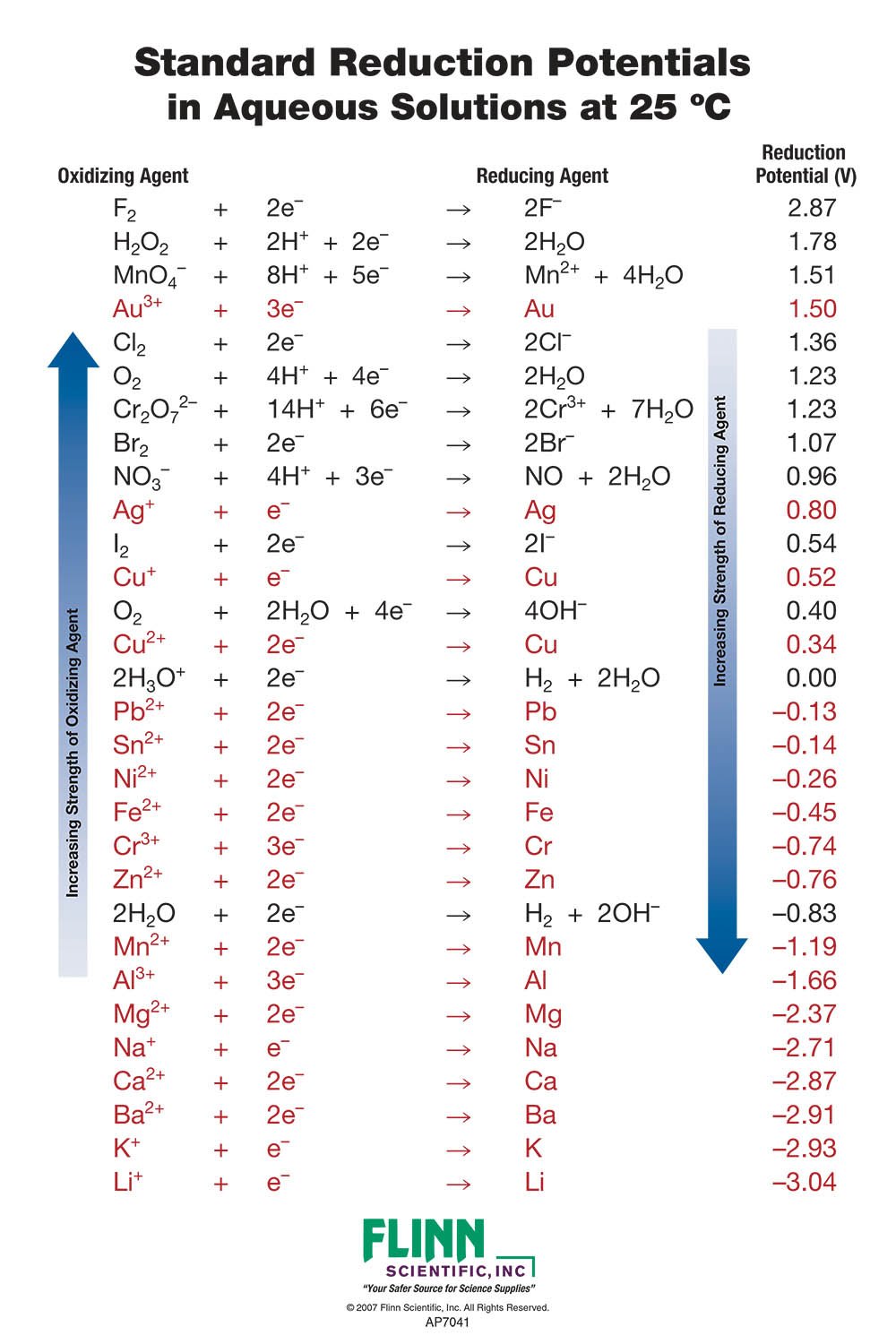
Table of Standard reduction potentials.pdf - Table of Standard reduction potentials Half reaction Li e Li s K e K s Ca2 2e Ca s Na e | Course Hero
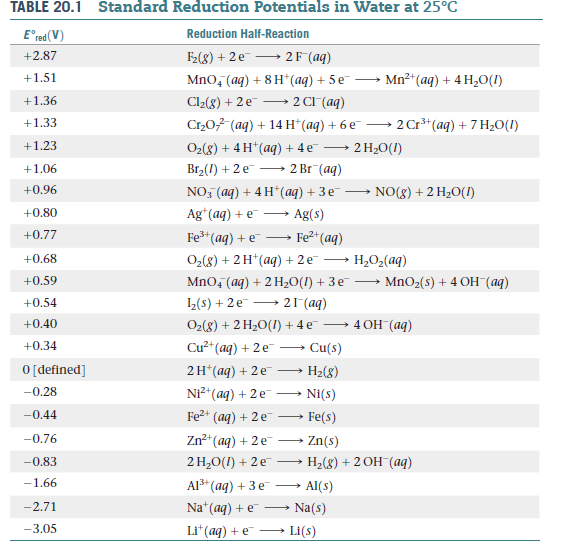
![SOLVED: Table of half-cell potentials: Potential (V) Reduction Half-Reaction +2.87 +1.51 +1.36 +1.33 +1.23 +1.06 +0.96 +0.80 +0.77 +0.68 +0.59 +0.54 +0.40 +0.34 0 [defined] -0.28 -0.44 -0.76 -0.83 -1.66 -2.71 -3.05 SOLVED: Table of half-cell potentials: Potential (V) Reduction Half-Reaction +2.87 +1.51 +1.36 +1.33 +1.23 +1.06 +0.96 +0.80 +0.77 +0.68 +0.59 +0.54 +0.40 +0.34 0 [defined] -0.28 -0.44 -0.76 -0.83 -1.66 -2.71 -3.05](https://cdn.numerade.com/ask_images/776fafd425914914b7523cc82f47d4b1.jpg)
SOLVED: Table of half-cell potentials: Potential (V) Reduction Half-Reaction +2.87 +1.51 +1.36 +1.33 +1.23 +1.06 +0.96 +0.80 +0.77 +0.68 +0.59 +0.54 +0.40 +0.34 0 [defined] -0.28 -0.44 -0.76 -0.83 -1.66 -2.71 -3.05

Table 1 from Potential-energy and free-energy surfaces of glycyl-phenylalanyl-alanine (GFA) tripeptide: experiment and theory. | Semantic Scholar

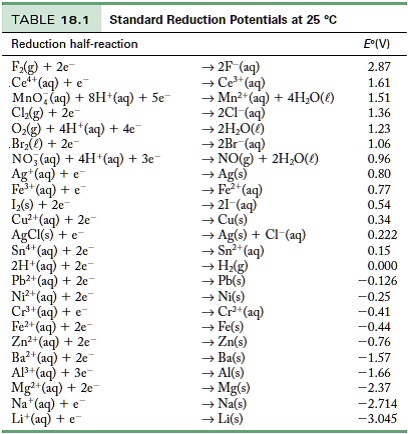
Using the standard electrode potentials given in the table, predict if the reaction between the following is possible. Ag^+(aq) and Cu(s)

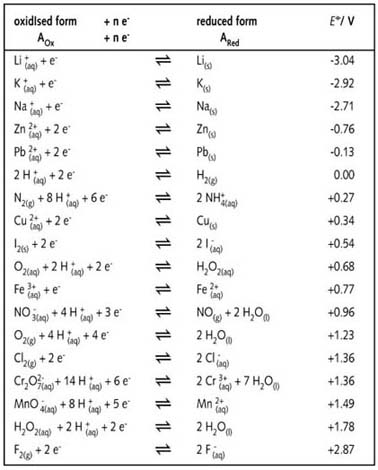
SOLVED: Table A5.5 Standard Reduction Potentials at 258C (298 K) for Many Common Half-Reactions Half-Reaction Half-Reaction 4OH Fz + 2e 2F 2.87 01 2H,0 + 4e Cu? + Ze Cu Ag + +

WebElements Periodic Table » Periodicity » Reduction potential of hydrated M(I) ions » Periodic table gallery

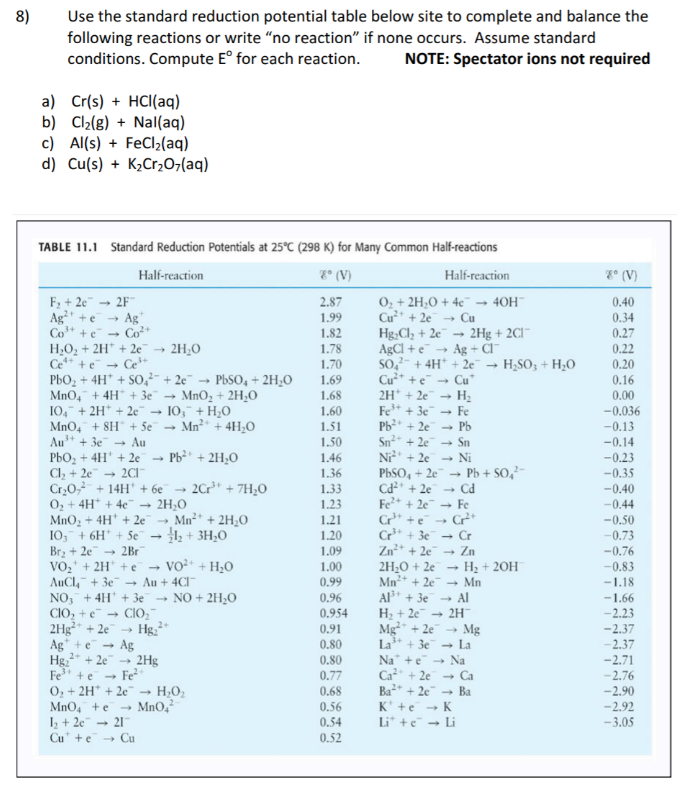
OneClass: Standard reduction potentials Use the table of standard reduction potentials given above to...