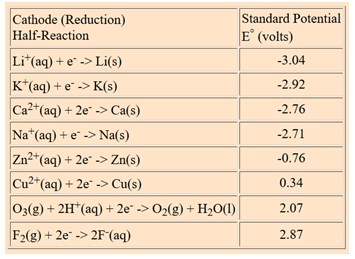
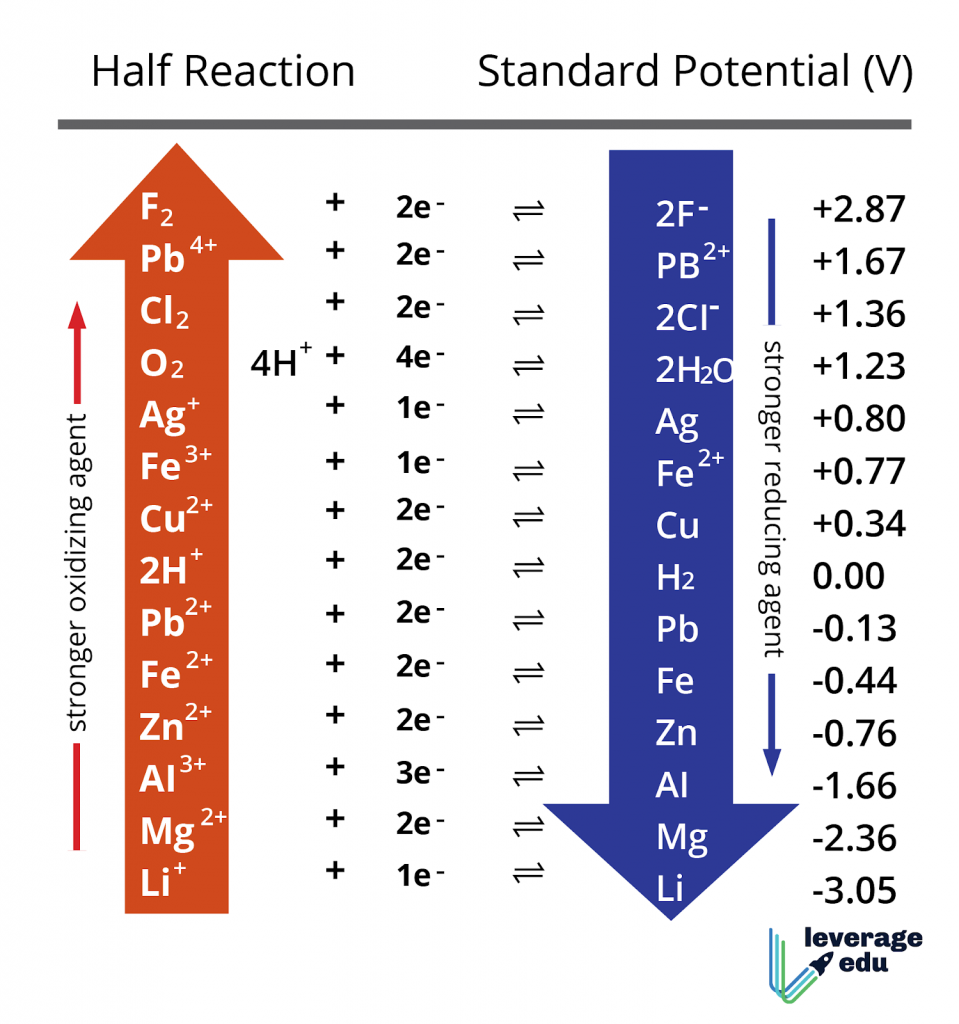
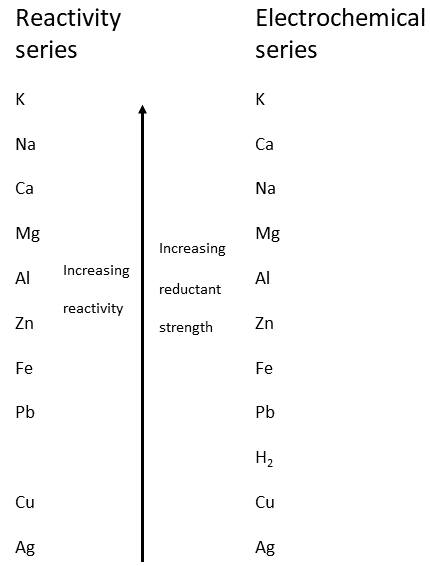
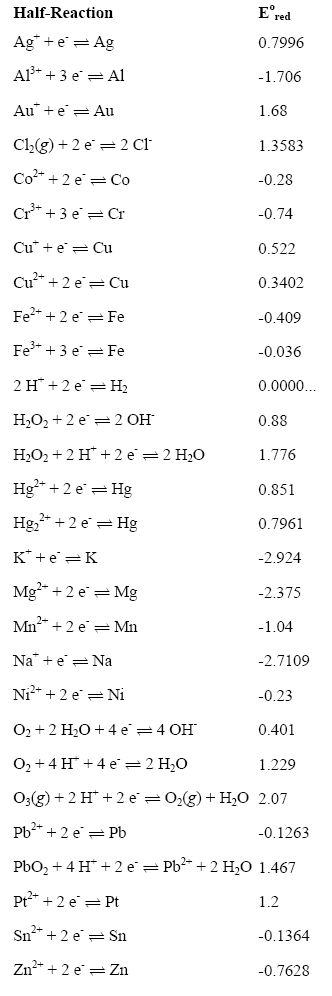![PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56964684a624c5af38c7e62256db3faa4c542d88/19-Table2-1.png)
PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar

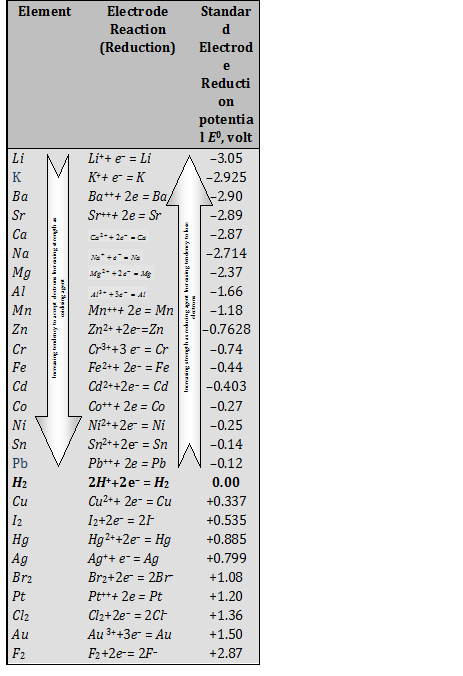
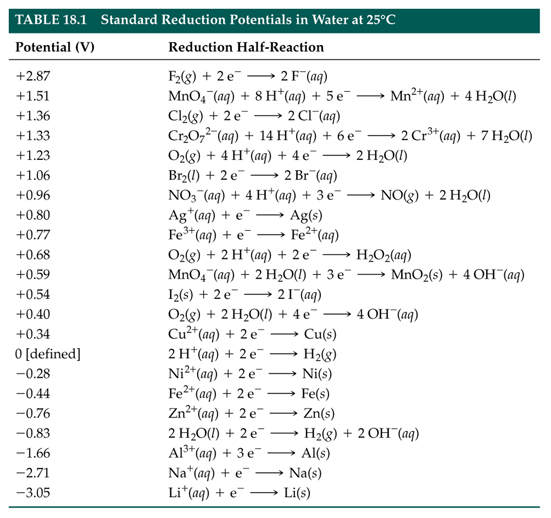
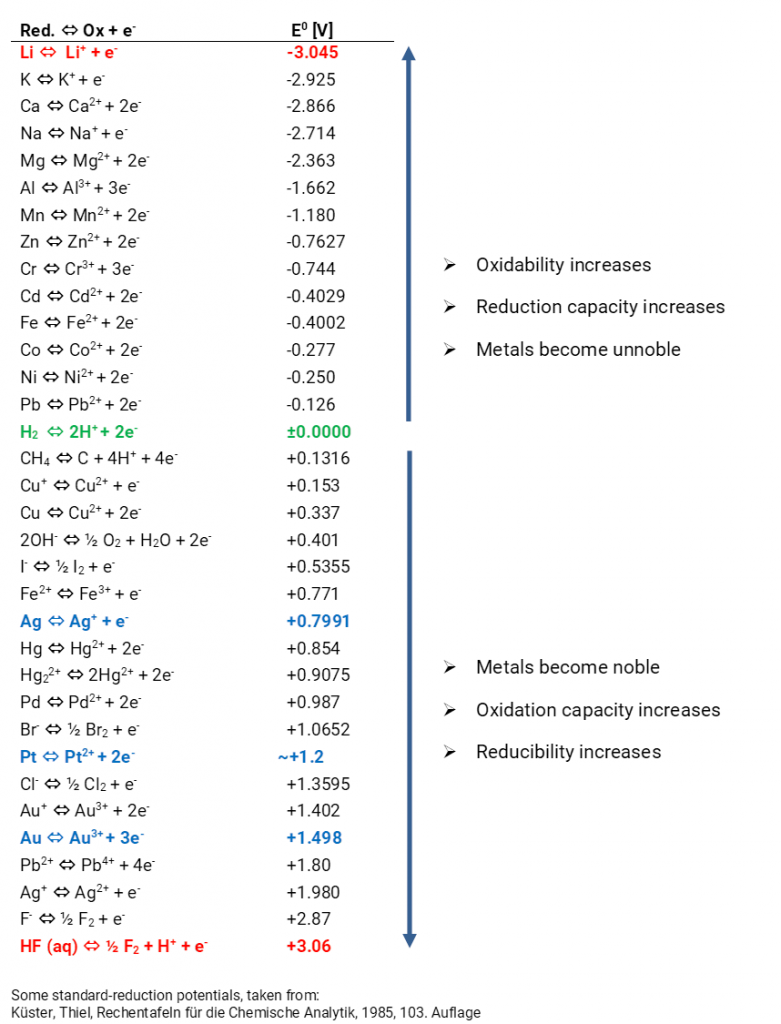
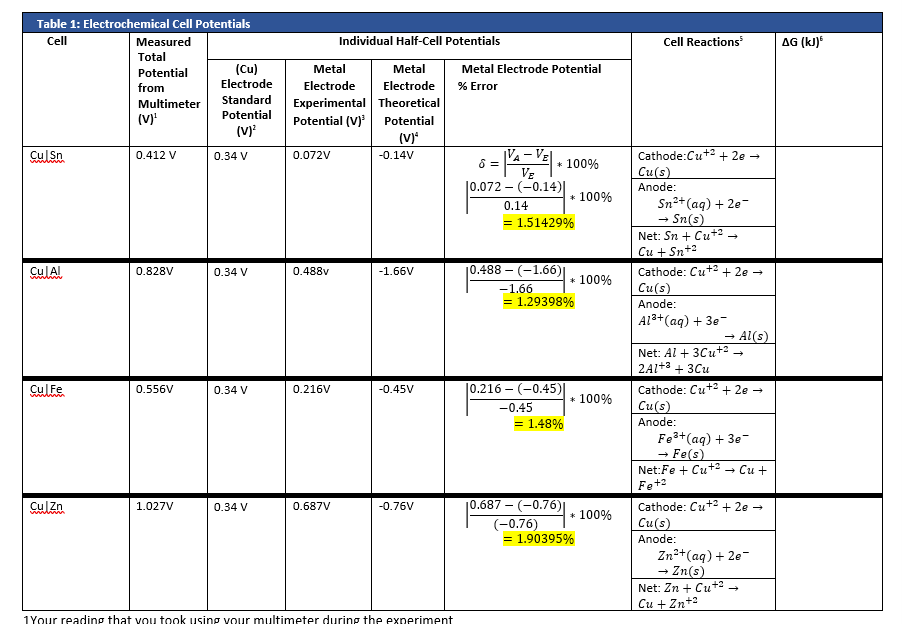
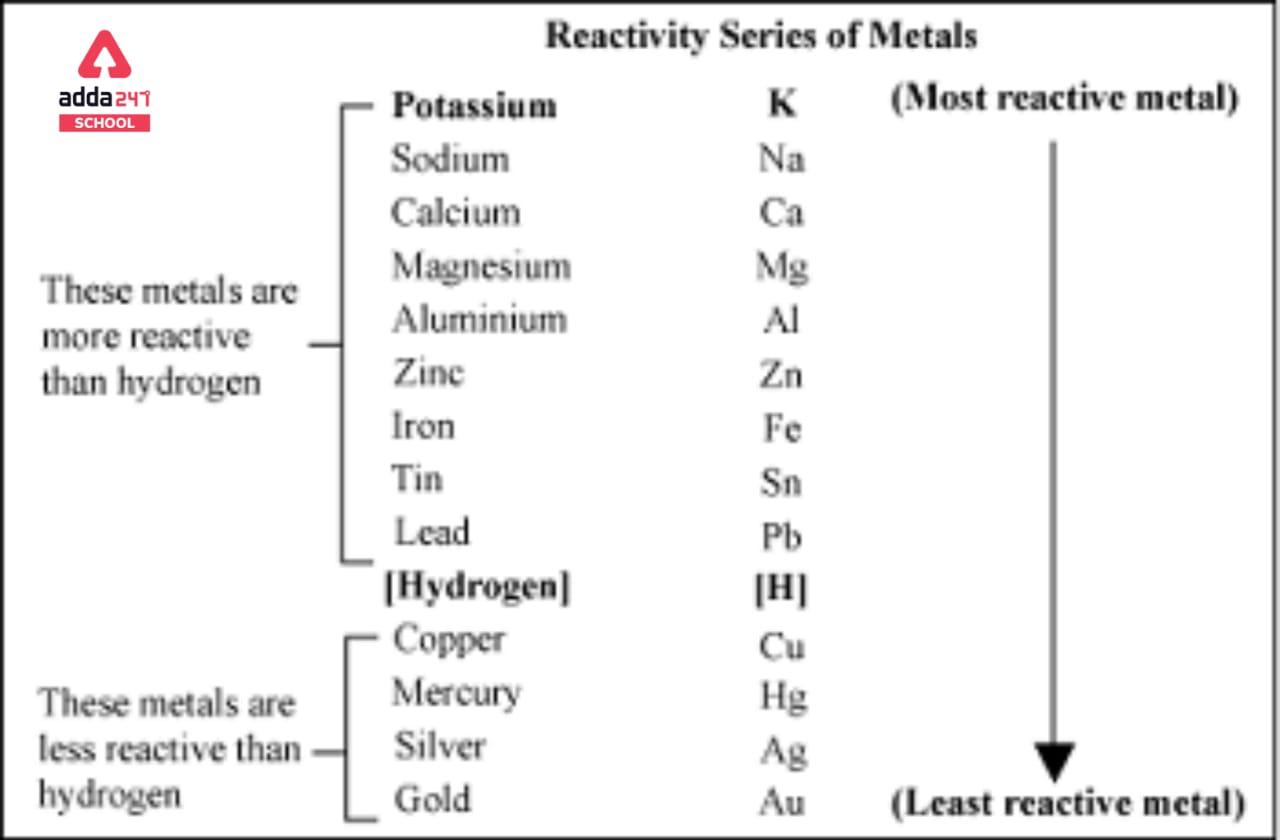
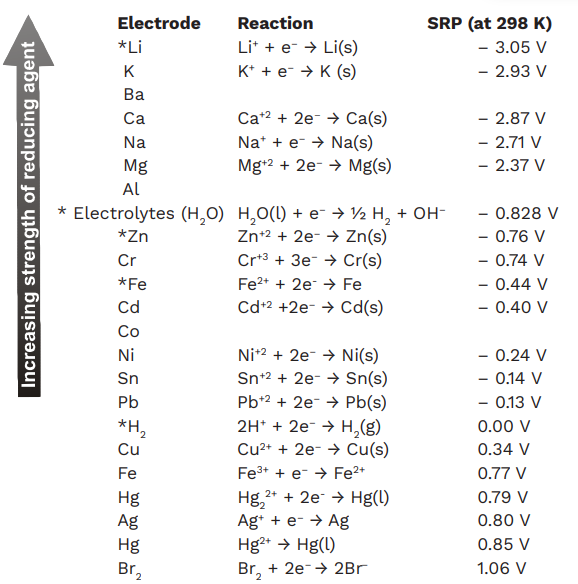
If for an electrode, the standard reduction potential is negative then, it is a than hydrogen electrode.

If for an electrode, the standard reduction potential is negative then, it is a than hydrogen electrode.






![The variation of the difference of the electrochemical potential [mV]... | Download Table The variation of the difference of the electrochemical potential [mV]... | Download Table](https://www.researchgate.net/publication/279312214/figure/tbl2/AS:667839455326213@1536236742592/The-variation-of-the-difference-of-the-electrochemical-potential-mV-between-the-coat.png)